Introduction
Hemophilia A is a rare X-linked bleeding disorder characterized by deficiency of the coagulation factor VIII (FVIII). The standard-of-care for hemophilia A includes prophylaxis (PPX) with factor replacement therapy or emicizumab to prevent bleeding. The World Federation of Hemophilia guidelines suggest FVIII trough levels of >3-5% or higher are adequate for PPX. While the importance of maintaining high FVIII levels is well recognized, real-world levels in patients with hemophilia A are often sub-optimal.
Aim
To understand unmet needs in patients with moderate or severe hemophilia A by assessing bleeding outcomes and patient-reported outcomes (PROs).
Methods
Patients from the United States (US) were recruited by PicnicHealth, a patient-centric data retrieval platform working with individuals who contribute their medical record data to research across various disease areas. Patients consented for PicnicHealth to retrieve their medical records from all care sites or providers and receive access to their records in the US. Patients were invited to complete bleed, treatment, and quality of life surveys. The analysis included medical record data (2015-2022) and PRO survey data (2022) from patients with moderate or severe hemophilia A (endogenous factor level ≤5%). Three cohorts were defined for the analysis of bleed outcomes, based on patients' PPX product class exposure (standard half-life [SHL] factor, extended half-life [EHL] factor, and emicizumab). Index date was defined as the first observed PPX product use within the class beginning on or after January 1, 2015, and patients were censored at the earliest of switch to another product class, end of patient data, death, or data cut-off (October 3, 2022). Patients who switched product classes during the study period were included in multiple cohorts. The PRO instrument used was the 29-item, 7-domain PROMIS-29 questionnaire (range 1-5; scale scores standardized to a general US adult population with mean [SD] score of 50 [10]). A custom instrument designed to assess patients' “worst pain intensity” was also used (from 0 = no pain to 10 = worst imaginable pain). An additional Medication and Bleed Survey was administered biweekly to collect patient-reported treatment and bleed event information.
Results
Of the 354 patients recruited, 293 had moderate or severe hemophilia A, of which 291 patients had a record of treatment. After excluding patients with inhibitors on index date or a non-hemophilia A bleeding disorder diagnosis on or prior to index date, 141 patients met the criteria for SHL, 88 for EHL, and 82 for emicizumab PPX cohorts (non-exclusive). Patients' mean (SD) age in years at index were as follows: SHL, 26.7 (13.3); EHL, 26.2 (11.8); and emicizumab, 29.8 (14.9). Considering only individual bleed events documented in medical records, 57.3% of the SHL cohort, 47.1% of the EHL cohort, and 55.2% of the emicizumab cohort had at least one recorded bleed within the first year post-index date. When also including bleed rates reported to the provider by the patient (for more complete data capture from the medical record) the respective values were 61.4%, 70.8% and 62.1%.
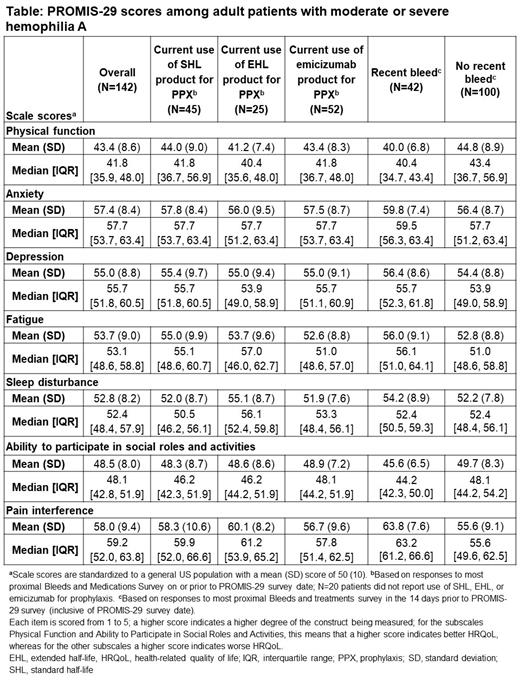
Out of 293 patients with moderate or severe hemophilia A, the PROMIS-29 questionnaire was completed by 142 patients who were ≥18 years of age at the time of survey completion. Scores were similar across patients' self-reported treatment class at the time of survey ( Table). Physical functioning and ability to participate in social roles and activities were the most affected areas; the most frequently reported symptoms were sleep disturbance (84.5%) and inability to participate in social roles and activities (69.7%). Adult patients completing the custom survey (n=140/293) rated their worst pain intensity as a mean (SD) of 7.7 (2.1); in patients with a recent bleed, this was 8.4 (1.5). Overall, patients reporting a recent bleed had worse scores.
Conclusion
This real-word study provides insights into the unmet needs in patients with hemophilia A receiving regular PPX. Irrespective of treatment received, a majority of patients still bleed at least once a year, struggle with pain and participating in social roles and activities, and have sleep disturbances. This indicates a need for improvement in the quality of life of this population and further highlights the need for understanding the patient perspective with hemophilia A.
Disclosures
Thornburg:Sanofi: Honoraria; Novo Nordisk: Research Funding; BioMarin: Research Funding. Wheeler:HEMA Biologics: Honoraria; Pfizer: Honoraria; Novo Nordisk: Honoraria; Genentech: Honoraria; UniQure: Honoraria; Octapharma: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; CSL Behring: Honoraria; Bayer: Honoraria. Bozzi:Sanofi: Current Employment, Current equity holder in publicly-traded company. Miles:Sanofi: Current equity holder in publicly-traded company; Sanofi: Current Employment. Hawaldar:Sanofi: Current Employment, Current equity holder in publicly-traded company. Wilson:Sanofi: Current Employment, Current equity holder in publicly-traded company. Hanson:PicnicHealth: Current Employment, Current equity holder in publicly-traded company. Liao:PicnicHealth: Current Employment. Wilson:Sanofi: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal